Il 23 Inhibitors

Biologics In Psoriasis The Next Generation Practical Dermatology

Current Model Of The Pathophysiology Of Psoriasis Il 23 Bridges The Download Scientific Diagram
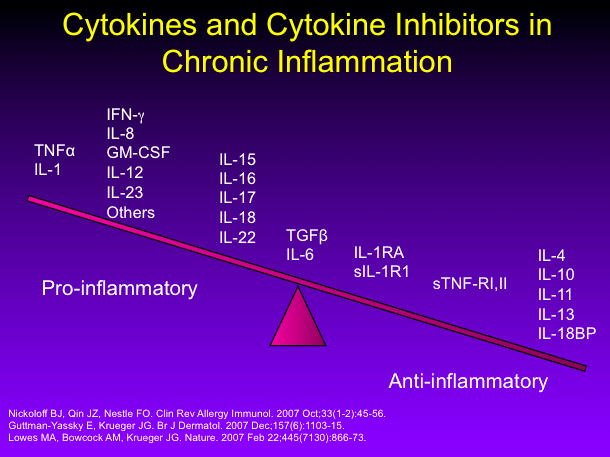
Systemic Treatment Of Psoriasis What S New Maui Derm
Assessing The Relative Efficacy Of Interleukin 17 And Interleukin 23 Targeted Treatments For Moderate To Severe Plaque Psoriasis A Systematic Review And Network Meta Analysis Of Pasi Response

A Balance Of Interleukin 12 And 23 In Cancer Trends In Immunology

Psoriasis Translating Current And Emerging Therapies Into Enhanced Management Strategies Youtube
IL‐23 is a heterodimer composed of two subunits p40, which is shared with IL‐12, and p19 3 Data from long‐term clinical trials and a large safety registry (Psoriasis Longitudinal Assessment and Registry;.
Il 23 inhibitors. To evaluate whether IL17, IL12/23 or TNF inhibitors are associated with an increased risk for serious infection in realworld patients with psoriasis or PsA, Li and colleagues conducted a. Evidence Review of Selective IL23 Inhibitors At this time, 2 agents that specifically target IL23 are approved by the FDA for the management of psoriasis Guselkumab (Tremfya) gained approval in July 17, with tildrakizumab (Ilumya) following in March 18. IL23 versus IL12/23 IL23 has been recognized as a major factor in the etiology and pathogenesis of psoriasis, and recent therapeutic development has focused on inhibition of the inflammatory.
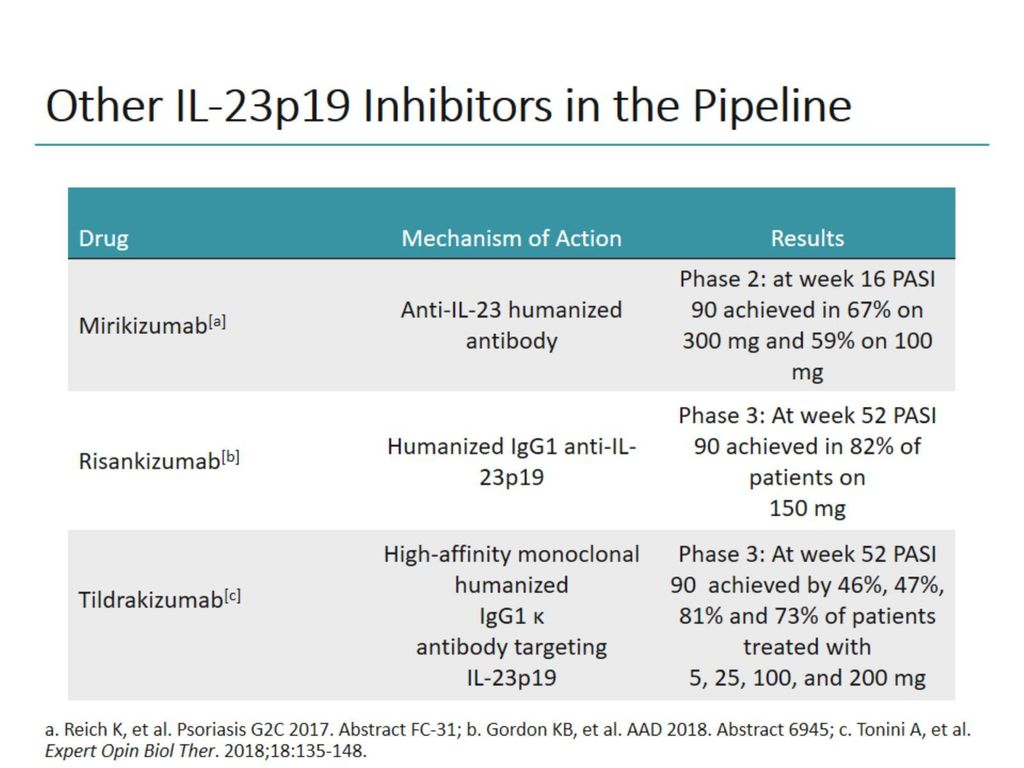
Three p19 IL23 inhibitors are approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) This report focusses specifically on these inhibitors, summarising the results of Phase III clinical trials for guselkumab, tildrakizumab, and risankizumab (Table 2) Their clinical efficacy, safety, and tolerability in. Although IL17 inhibitors are prescribed more often than IL23 inhibitors when joint involvement is a factor, the IL23 class is gaining momentum In July , Janssen’s Tremfya became the first IL23 inhibitor to gain FDA approval for the treatment of moderate to severe psoriatic arthritic (PsA). IL12/23 Inhibitors and Small Molecule Inhibitors;.
The IL23 inhibitors’ mechanism of action and infrequent dosing schedule provide advantages over previousgeneration biologics, says Mark Lebwohl, MD He is the Waldman Professor and Chairman, Kimberly and Eric J Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai. Interleukin 23 (IL23) Inhibitors Ilumya (tildrakizumabasmn), Skyrizi (risankizumabrzaa) and Tremfya (guselkumab) work by targeting interleukin 23 (IL23) This cytokine is linked with inflammation in psoriasis and PsA Ilumya, Skyrizi and Tremfya work to reduce psoriatic symptoms and slow disease progression. Interleukin6 Inhibitors Last Updated August 27, Interleukin (IL)6 is a pleiotropic, proinflammatory cytokine produced by a variety of cell types, including lymphocytes, monocytes, and fibroblasts.
Three p19based IL23 inhibitors are currently approved for psoriasis guselkumab (first approved), tildrakizumab, and risankizumab (most recently approved) This study assessed the relative benefits and risks of these drugs using adalimumab as a common comparator. IL12/23 Inhibitors and Small Molecule Inhibitors;. The trouble, though, is that their drug was clearly overshadowed by guselkumab, another IL23 drug that’s now angling to hit the market to compete against two other recent arrivals.
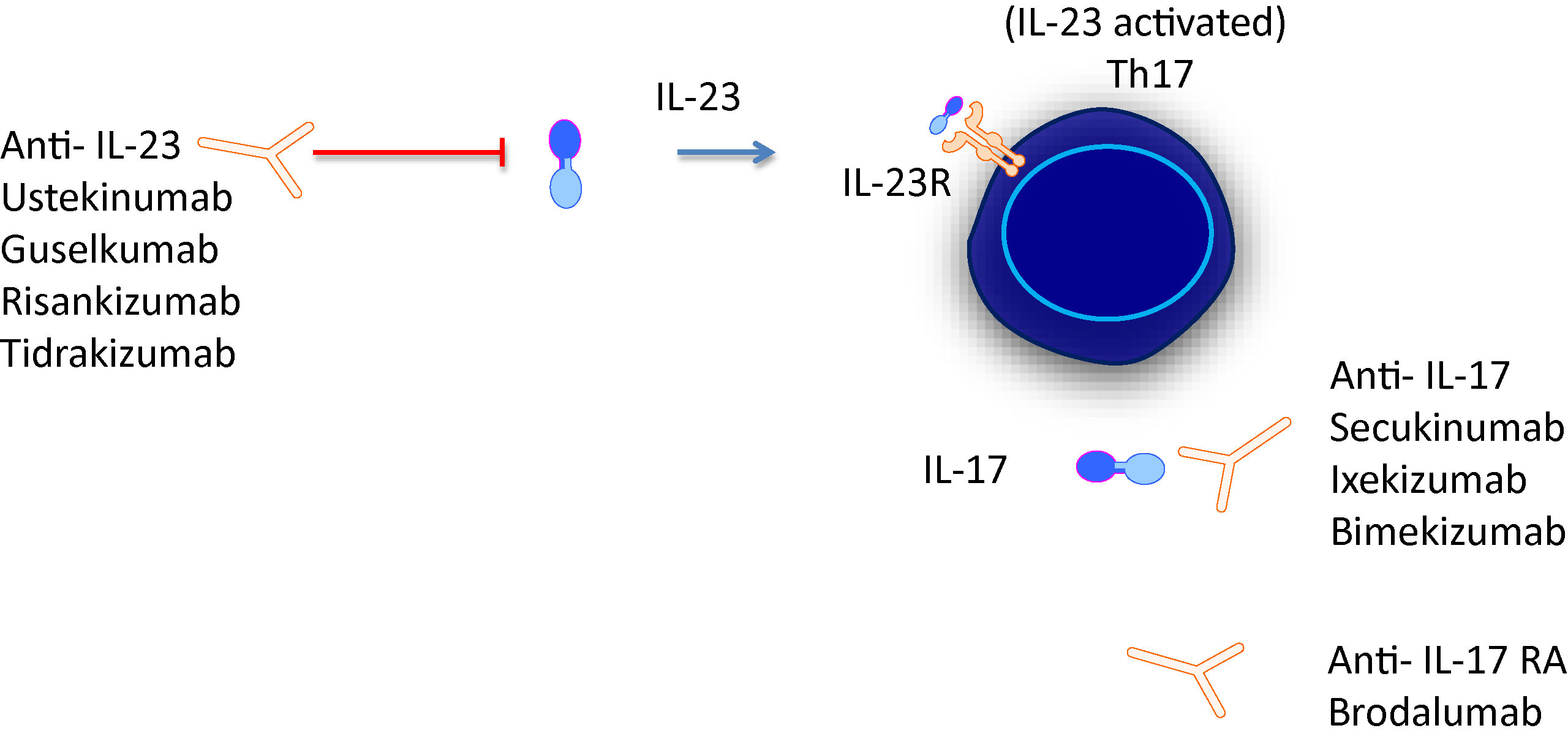
Biologic medications for psoriasis Antiinterleukin17 (IL17) agents Interleukin17 (IL17) is a cytokine, a type of immune protein It induces IL12/23 inhibitors IL12/23 inhibitors target a subunit that’s shared by the IL12 and IL23 cytokines Both cytokines IL23 inhibitors IL23. IL23 Inhibitors Take Over the US Psoriasis Switching Market at the Expense of the IL17 and TNF Inhibitors AbbVie's Skyrizi now garners the greatest switchto rate, capturing the majority of new. Several drugs targeting the IL23/IL17 axis have been successfully tested in PsO and Ps For example, ustekinumab and secukinumab, inhibitors of IL12/IL23p40 and IL17A respectively, are recommended as a secondline biological treatment for PsA patients inadequate responders to conventionalsynthetic (cs) diseasemodifying antirheumatic.
IL12/IL23 inhibitors the advantages and disadvantages of this novel approach for the treatment of psoriasis Bartlett BL(1), Moody MN, Tyring SK Author information (1)Center for Clinical Studies, Houston, TX, USA. “Tremfya is the first and only selective IL23 inhibitor approved for both active psoriatic arthritis and moderate to severe plaque psoriasis, as well as the only biologic approved for the. IL23 inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and Janus kinase (JAK) inhibitors are currently encouraging further research Two drugs which are IL23 inhibitors are now in phase III of clinical trials The aim of the action of both drugs is selective IL23 inhibition by targeting the p19 subunit.
Because of its ability to affect IL17 production via IL23 without blocking IL17 activity, IL23 inhibitor biologics (Table) may be advantageous in the treatment of psoriasis These biologics may provide more efficacy, convenience, and safety than other biologics for certain populations of patients with psoriasis 1 2. USE USE for SKYRIZI® (risankizumabrzaa) SKYRIZI is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy). In July , Janssen's Tremfya became the first IL23 inhibitor to gain FDA approval for the treatment of moderate to severe psoriatic arthritic (PsA).
Blocking IL23 can slow clinical manifestation of psoriasis indirectly affecting Th17 immune response and producting of IL17 For treating of psoriasis is better to use ixekizumab which is IL17A antagonist because its faster than guselkumab, tildrakizumab or riskankizumab which are inhibitors of p19 of IL23. This humanized IgG1/k monoclonal antibody selectively binds to the p19 subunit of IL23 and inhibits its interaction with the IL23 receptor IL23 is a natural cytokine associated with. While IL17 inhibitors require dosing every 2–4 weeks, 32,33,44 IL12/23 and IL23 inhibitors are dosed less frequently, typically every 8–12 weeks 4648 In summary, while IL17 and IL23 inhibitors both represent highly efficacious and broadly welltolerated classes of therapy for psoriasis, 43 differences exist between agents in.
Antibodies that inhibit IL12/23 or IL23 are key treatment options for patients with psoriasis IL12 and IL23 also play a key role in immune responses to infections and tumors A growing body of information from clinical trials, cohort studies, postmarketing reports, genetic studies and animal models provides insights into the potential. The cytokines interleukin (IL)12 and interleukin (IL)23 have been impli cated variously in the pathogenesis of psoriasis and psoriatic arthritis (PsA). Request PDF Advancements in Biologic Therapy for Psoriasis the IL23 Inhibitors Purpose of Review The purpose of this article is to review clinical trials evaluating the efficacy and safety.
Update in Treatment of Psoriatic Arthritis;. IL23 Inhibitor Effective in Crohn's Disease Save Selective IL23 inhibition has been touted to be the next great advance in the treatment of inflammatory bowel and psoriatic disease The annual Digestive Disease Week (DDW) conference in San Diego reported early reults from a proofofconcept, Phase II study, wherein risankizumab (antiIL23) was shown to be more effective than placebo in patients with moderatelytoseverely active Crohn's disease. Update in Treatment of Psoriatic Arthritis;.
IL23 Inhibitors Generic Name Therapeutic Class or Brand Name IL23 Inhibitors Applicable Drugs (if Therapeutic Class) Guselkumab (Tremfya®), Risankizumab (Skyrizi™), Tildrakizumab (Ilumya™) GPI Code , , Preferred Risankizumab (Skyrizi™) Nonpreferred Guselkumab (Tremfya®), Tildrakizumab (Ilumya™). IL23 Inhibitors Generic Name Therapeutic Class or Brand Name IL23 Inhibitors Applicable Drugs (if Therapeutic Class) Guselkumab (Tremfya®), Risankizumab (Skyrizi™), Tildrakizumab (Ilumya™) GPI Code , , Preferred Risankizumab (Skyrizi™). The study, “ Risankizumab, an IL23 inhibitor, for ankylosing spondylitis results of a randomised, doubleblind, placebocontrolled, proofofconcept, dosefinding phase 2 study,” was published in the journal Annals of the Rheumatic Disease.
Interleukin23 (IL23) inhibitors are an important new class of drugs for the treatment of Crohn disease (CD) and ulcerative colitis (UC), both common causes of inflammation of the digestive tract Johnson & Johnson’s Stelara (ustekinumab) is the only IL23 inhibitor currently approved to treat moderatetosevere CD and UC in the United States. Herein, we describe the treatment of 2 patients with suberythrodermic pityriasis rubra pilaris (PRP) with guselkumab, a specific interleukin 23 (IL23)p19 inhibitor View. IL23 Inhibitors Take Over the US Psoriasis Switching Market at the Expense of the IL17 and TNF Inhibitors Read full article November 17, , 500 AM · 4 min read.
The IL23 inhibitors’ mechanism of action and infrequent dosing schedule provide advantages over previousgeneration biologics, says Mark Lebwohl, MD He is the Waldman Professor and Chairman, Kimberly and Eric J Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai. Request PDF Advancements in Biologic Therapy for Psoriasis the IL23 Inhibitors Purpose of Review The purpose of this article is to review clinical trials evaluating the efficacy and safety. Therapies such as ustekinumab and guselkumab inhibit IL23 Ustekinumab targets the p40 subunit common to both IL23 and IL12 while guselkumab targets the p19 subunit found in IL23 IL17 subtypes trigger downstream inflammation in psoriasis.
Ustekinumab (Stelara) is an IL12/23 inhibitor that’s FDpproved to treat psoriasis IL23 inhibitors These inhibitors can then effectively block the protein from carrying out its function. The final biologic is mirikizumab (LY, Eli Lilly), which is still in clinical trials “This medication is given once every four weeks,” Dr Lebwohl said “It appears to be quite effective, although it is administered slightly more often than the other three antiIL23 biologics”. Interleukin (IL)17 and IL23 inhibitors are associated with a relatively low incidence of adverse effects (AEs) when used for the treatment of psoriasis and psoriatic arthritis, study data published in the Journal of the European Academy of Dermatology and Venereology suggest.
ICER IL23 Inhibitors Are Preferable to AntiTNF Agents for Plaque Psoriasis The Institute for Clinical and Economic Review (ICER) explains that, compared with anti–tumor necrosis factor (antiTNF) drugs, both guselkumab and risankizumab offered a superior benefit based on currently available data. Interleukin6 Inhibitors Last Updated August 27, Interleukin (IL)6 is a pleiotropic, proinflammatory cytokine produced by a variety of cell types, including lymphocytes, monocytes, and fibroblasts. Types of IL23 inhibitor Guselkumab (Tremfya) Tremfya is a type of selfinjectable IL23 inhibitor People who take this medication can inject Risankizumabrzaa (Skyrizi) Skyrizi is another type of selfinjectable IL23 inhibitor People who take Skyrizi can Tildrakizumabasmn (Ilumya).
IL23 inhibitors represent the latest class of therapies to emerge, adding to already available agents, which include TNF inhibitors, IL12/23 inhibitors, and IL17 inhibitors Given the spectrum of potential treatment options available, it is important to understand the role and importance of each class of agent in the therapeutic armamentarium. PSOLAR) have shown the IL‐12/23p40 inhibitor ustekinumab to be well tolerated in patients with psoriasis 2226 However, another. IL12 and IL23 inhibitors remain on the forefront of treatment options for inflammatory diseases such as psoriasis, Crohn’s disease, multiple sclerosis, and rheumatoid arthritis Although the current data does not provide insight into the longterm effects of these drugs, results have been extremely encouraging.
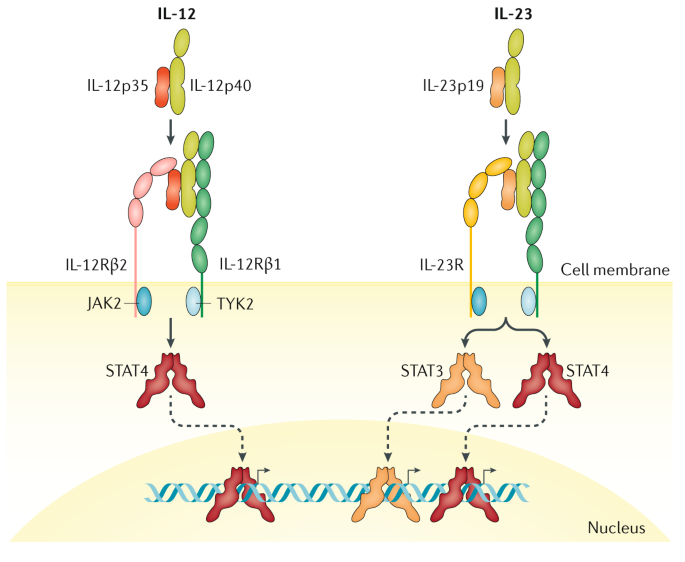
IL23 Inhibitors and Other Emerging Psoriasis Treatments;. IL‐23 inhibitors in development Three inhibitors specifically targeting IL‐23p19 are currently in active development for the treatment of moderate‐to‐severe psoriasis (Table 2) tildrakizumab, guselkumab and risankizumab A further antibody, LY (mirikizumab), is now entering phase 2 development 124. IL12 is a heterodimer composed of a p40 and p35 subunit Research showed that the p40 subunit of IL12 also paired with a unique p19 subunit This new, additional cytokine was classified as IL23, 11 and it was later discovered to play a more central role in psoriasis than IL12 9 Thus, research shifted from the inhibition of IL12 to IL23.
Interleukin inhibitors are immunosuppressive agents which inhibit the action of interleukins Interleukins are a group of cytokines which are synthesized by lymphocytes, monocytes, macrophages, and certain other cells They function especially in regulation of the immune system. Introduction Blockers of IL12/23, as well as specific blockers of IL23, have been investigated as options for medical therapy in inflammatory bowel disease. The monoclonal antibodies tildrakizumab, guselkumab and risankizumab target the p19 subunit that is specific to interleukin (IL)‐23 This article reviews published data on the safety of these IL‐23p19 inhibitors in patients with psoriasis compared with other currently available biologic therapies.
IL23 Inhibitors Take Over the US Psoriasis Switching Market at the Expense of the IL17 and TNF Inhibitors AbbVie's Skyrizi now garners the greatest switchto rate, capturing the majority of new. “Tremfya is the first and only selective IL23 inhibitor approved for both active psoriatic arthritis and moderate to severe plaque psoriasis, as well as the only biologic approved for the. Interleukin23 inhibitor outperforms Johnson & Johnson's blockbuster New data from a phase II trial of Boehringer Ingelheim's psoriasis candidate BI back up earlier results showing it is more effective than a rival drug from Johnson & Johnson After nine months' treatment, 69% of moderatetosevere plaque patients treated with the highest dose (180mg) of Boehringer's interleukin23 inhibitor had clear or almost clear skin, compared to 30% of patients treated with J&J's Stelara.
Update in Traditional Treatments (Topicals, UV Phototherapy, Systemics). At this time, 2 inhibitors of IL23 p19 have been approved by the United States Food and Drug Administration, guselkumab and tildrakizumab Two other agents, risankizumab and mirikizumab, have completed phase 3 and phase 2 of development, respectively Pivotal trials in the development of these agents and clinical use of the approved agents are discussed. Request PDF Advancements in Biologic Therapy for Psoriasis the IL23 Inhibitors Purpose of Review The purpose of this article is to review clinical trials evaluating the efficacy and safety.
Request PDF Advancements in Biologic Therapy for Psoriasis the IL23 Inhibitors Purpose of Review The purpose of this article is to review clinical trials evaluating the efficacy and safety. Update in Traditional Treatments (Topicals, UV Phototherapy, Systemics). Because of its ability to affect IL17 production via IL23 without blocking IL17 activity, IL23 inhibitor biologics (Table) may be advantageous in the treatment of psoriasis These biologics may provide more efficacy, convenience, and safety than other biologics for certain populations of patients with psoriasis.
Recent studies have suggested that a molecule involved in immune inflammatory processes, called IL23, may be targeted as a potential treatment strategy for AS AbbVie researchers have developed an antibody, risankizumab (BI /ABBV066), that can selectively inhibit IL23 Risankizumab has been shown beneficial in other inflammatory diseases such as psoriasis, psoriatic arthritis (PsA), and Crohn’s disease. “In animal models, blocking IL23 does not appear to cause cancer either,” he said Nonetheless, data shows that at least for guselkumab and tildrakizumab, efficacy is slightly less efficacious in heavier patients. Google Scholar Interleukin 23 is a heterodimeric cytokine composed of two subunits p40, which is also a subunit of interleukin 12 and is targeted by ustekinumab (a biological drug approved for psoriasis and psoriatic arthritis), and p19, which is expressed in interleukin 23 only Likewise, the development of specific inhibitors of interleukin 23 followed the design of human or humanised monoclonal antibodies against p19.
TNFInhibitors “Classic” Biologics for Psoriatic Disease;. An IL12/23 inhibitor may be effective when PsA has not responded well to TNF inhibitors or IL17 inhibitors Alternately, a doctor may prescribe an IL12/23 inhibitor if a person has both PsA and. Interleukin 23 (IL23) Inhibitors Ilumya (tildrakizumabasmn), Skyrizi (risankizumabrzaa) and Tremfya (guselkumab) work by targeting interleukin 23 (IL23) This cytokine is linked with inflammation in psoriasis and PsA Ilumya, Skyrizi and Tremfya work to reduce psoriatic symptoms and slow disease progression.
Currently, guselkumab, risankizumab, and tildrakizumab are the IL23 inhibitors approved in the United States and European Union Additionally, it’s expected that mirikizumab may be approved within the next couple of years These inhibitors have shown efficacy, safety, and a maintenance of response in clinical trials. To date, the FDA have approved two IL17 inhibitors for PsA ixekizumab (Taltz) secukinumab (Cosentyx).
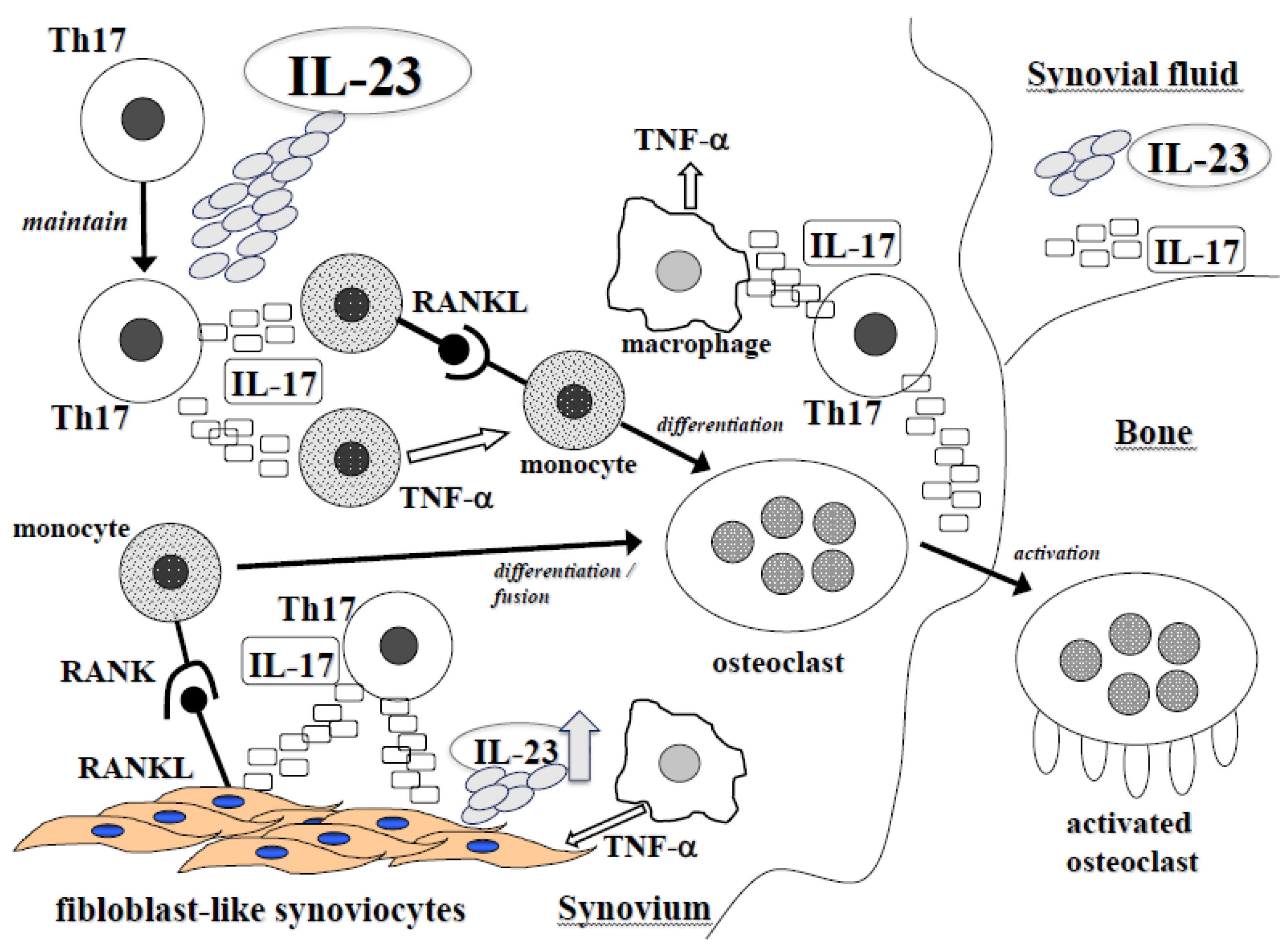
Jcm Free Full Text Il 23 And Th17 Disease In Inflammatory Arthritis Html
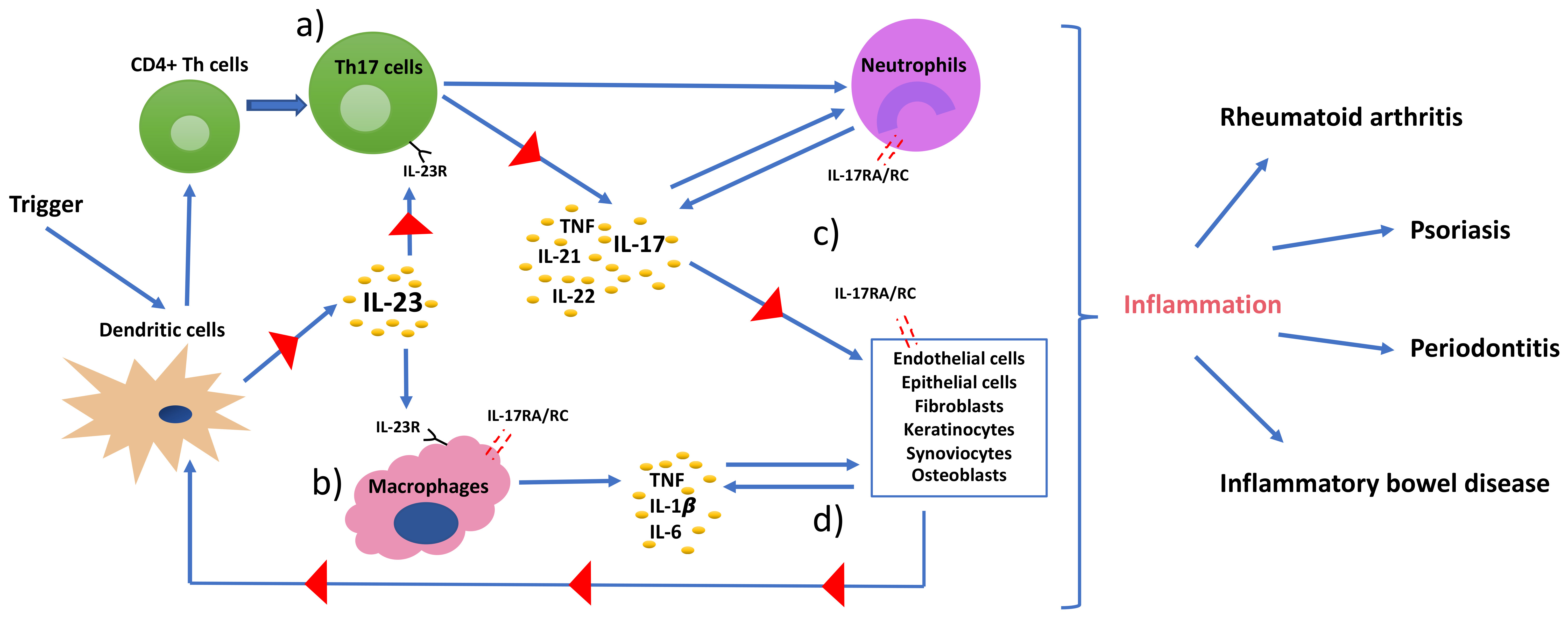
Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html
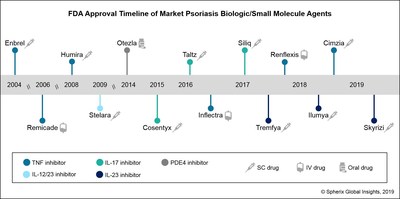
Robust Use Of Janssen S Tremfya And Positive Early Launch Metrics For Abbvie S Skyrizi May Threaten Growth Of Il 17 Inhibitors In Us Psoriasis Market According To Spherix Global Insights Markets Insider
Evolution Of Treatment Strategies Targeting Il 23 For Psoriasis Ppt Download

Tofacitinib Attenuates Pathologic Immune Pathways In Patients With Psoriasis A Randomized Phase 2 Study Journal Of Allergy And Clinical Immunology
Www Jaad Org Article S0190 9622 18 X Pdf
Www Cell Com Cell Reports Pdf S2211 1247 18 0 Pdf
Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology
Effects Of The Il 23 Il 17 Pathway On Bone In Spondyloarthritis Nature Reviews Rheumatology
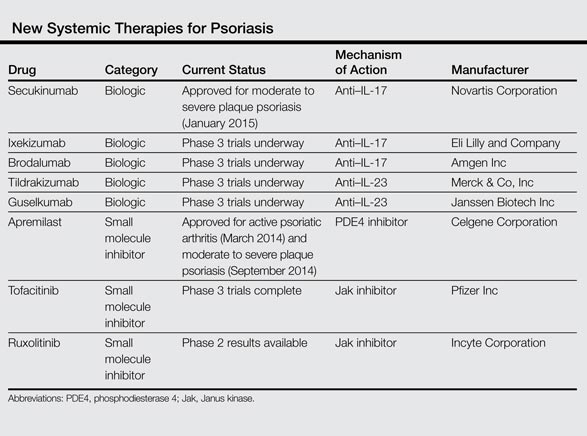
New Systemic Therapies For Psoriasis Mdedge Dermatology

Systemic Inflammation In Psoriasis A Guide For Dermatology Care Providers Practical Dermatology
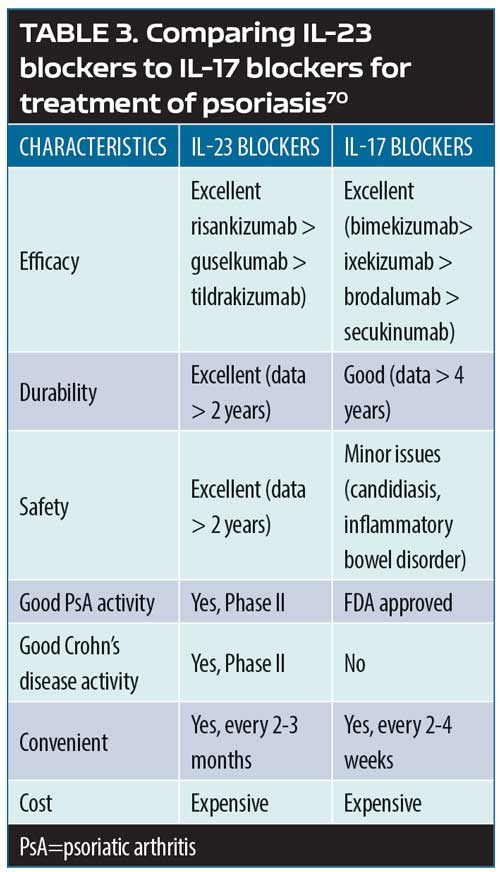
Update On The Pathogenesis Of Psoriasis A Bench To Bedside Success Story Maui Derm

Full Text Current Perspective On The Role Of The Interleukin 23 Interleukin 17 A Itt

The Il 23 T17 Pathogenic Axis In Psoriasis Is Amplified By Keratinocyte Responses Sciencedirect
Http Link Springer Com Content Pdf 10 1007 2f978 3 0348 06 6 232 1 Pdf

Monoclonal Antibodies For The Treatment Of Plaque Psoriasis Prescriberprescriber
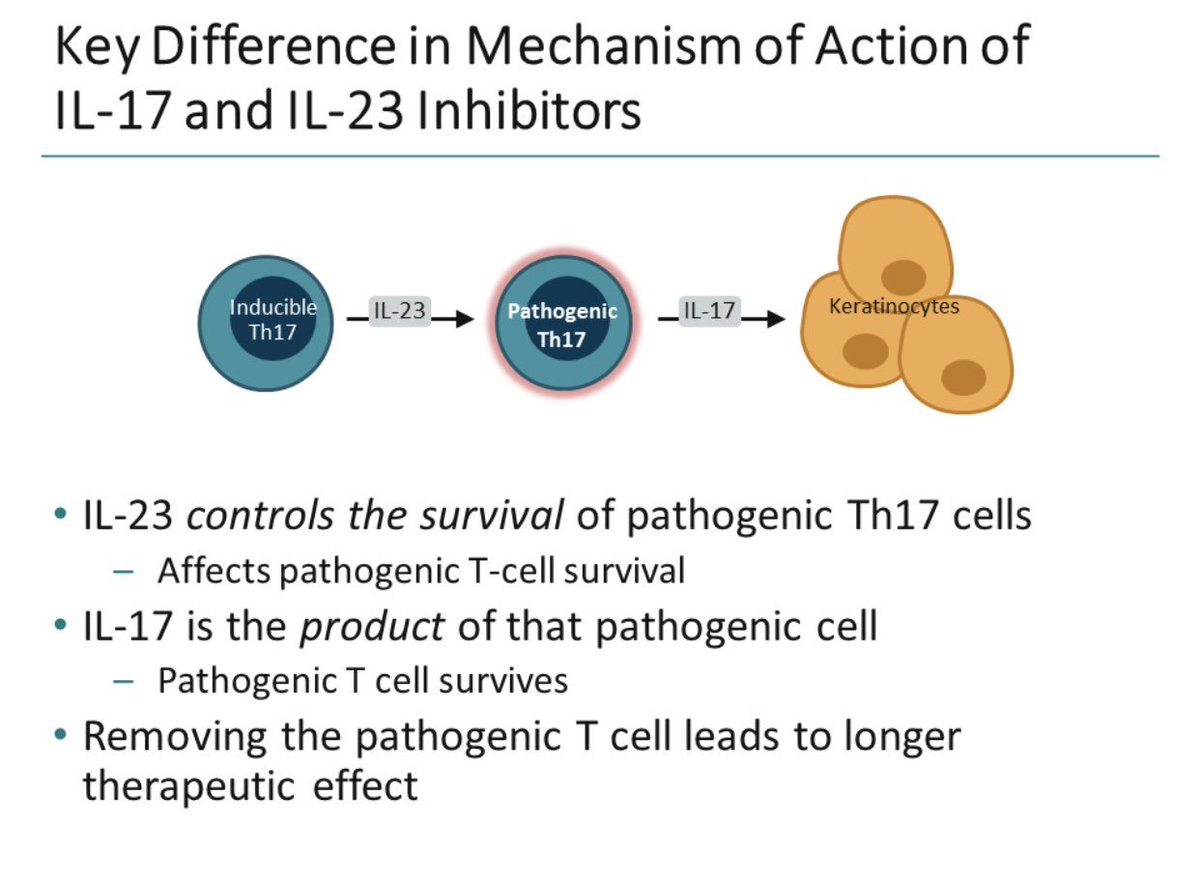
Dr Hanady Manasfi Key Difference In Mechanism Of Action Of Il 17 And Il 23 Inhibitors In Psoriasis
2

Psoriasis Treatment Unmet Needs Present 19 Opportunities Pm360

Mirikizumab An Overview Sciencedirect Topics

The Role Of Il 23 And The Il 23 Th17 Immune Axis In The Pathogenesis And Treatment Of Psoriasis Girolomoni 17 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library
Q Tbn And9gcr3mhz6mcjy6jek4cw2jcumiwk Oyqmbe3r74bs4ois7cvvf9 O Usqp Cau
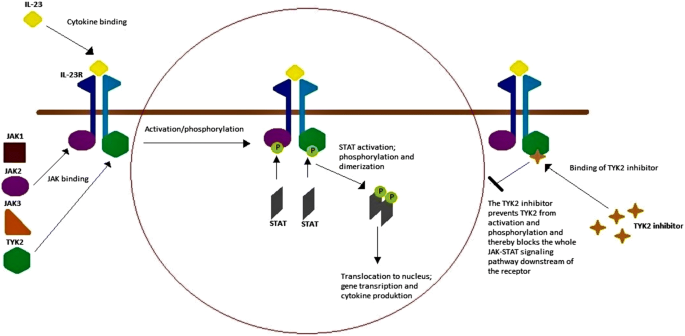
Systemic Treatment Of Psoriasis With Jak Inhibitors A Review Springerlink

Il 23 Inhibition In Psoriasis A Novel Approach To Convenient Consistent Clearance European Medical Journal
Www Caresource Com Documents Srx 0043

Pdf Short Term Efficacy And Safety Of Il 17 Il 12 23 And Il 23 Inhibitors Brodalumab Secukinumab Ixekizumab Ustekinumab Guselkumab Tildrakizumab And Risankizumab For The Treatment Of Moderate To Severe Plaque Psoriasis A Systematic Review And

Risankizumab Versus Ustekinumab For Moderate To Severe Plaque Psoriasis Nejm
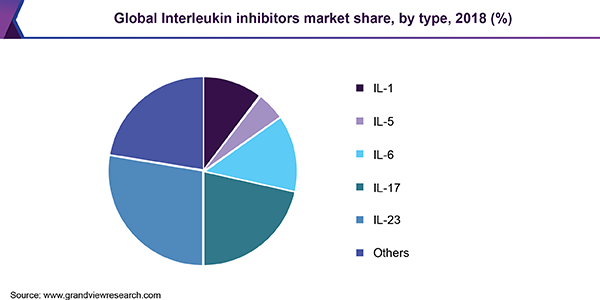
Il Inhibitors Market Size Share Industry Trends Report 19 26
Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html

Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study The Lancet

Putting Together The Psoriasis Puzzle An Update On Developing Targeted Therapies Disease Models Mechanisms

Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis Results Of A Randomised Double Blind Placebo Controlled Proof Of Concept Dose Finding Phase 2 Study Annals Of The Rheumatic Diseases

Figure 1 From Selective Interleukin 23 P19 Inhibition Another Game Changer In Psoriasis Focus On Risankizumab Semantic Scholar
Q Tbn And9gcsyddolyfjahkrlafzrifazci2kbymbcx4o4jp4bfjulqyevxq9 Usqp Cau

Il 23 Inhibitors For Treating Psoriasis What To Know

Inside The New Psoriasis Guidelines Part I Population Health Learning Network

Po 1wpapdcrn6m
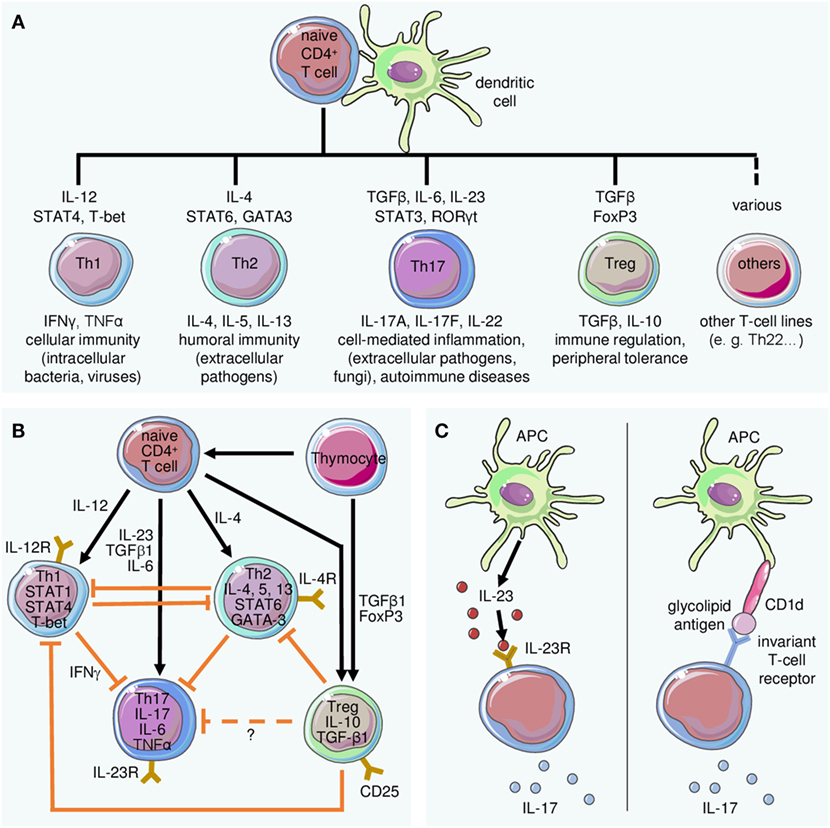
Frontiers The Interleukin 23 Interleukin 17 Axis Links Adaptive And Innate Immunity In Psoriasis Immunology

Figure 1 Th17 Associated Cytokines As A Therapeutic Target For Steroid Insensitive Asthma
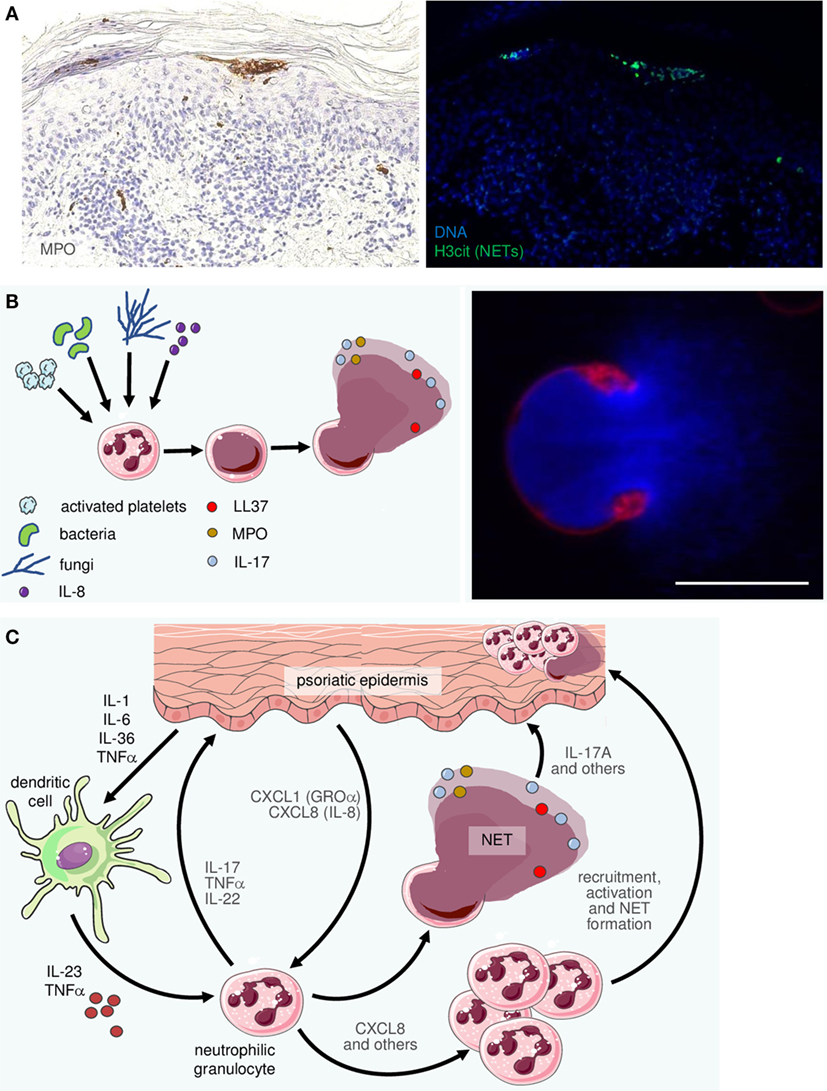
Frontiers The Interleukin 23 Interleukin 17 Axis Links Adaptive And Innate Immunity In Psoriasis Immunology

Novel Il 23 Targeted Inhibitors For Psoriasis Treatment Ppt Download

Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology
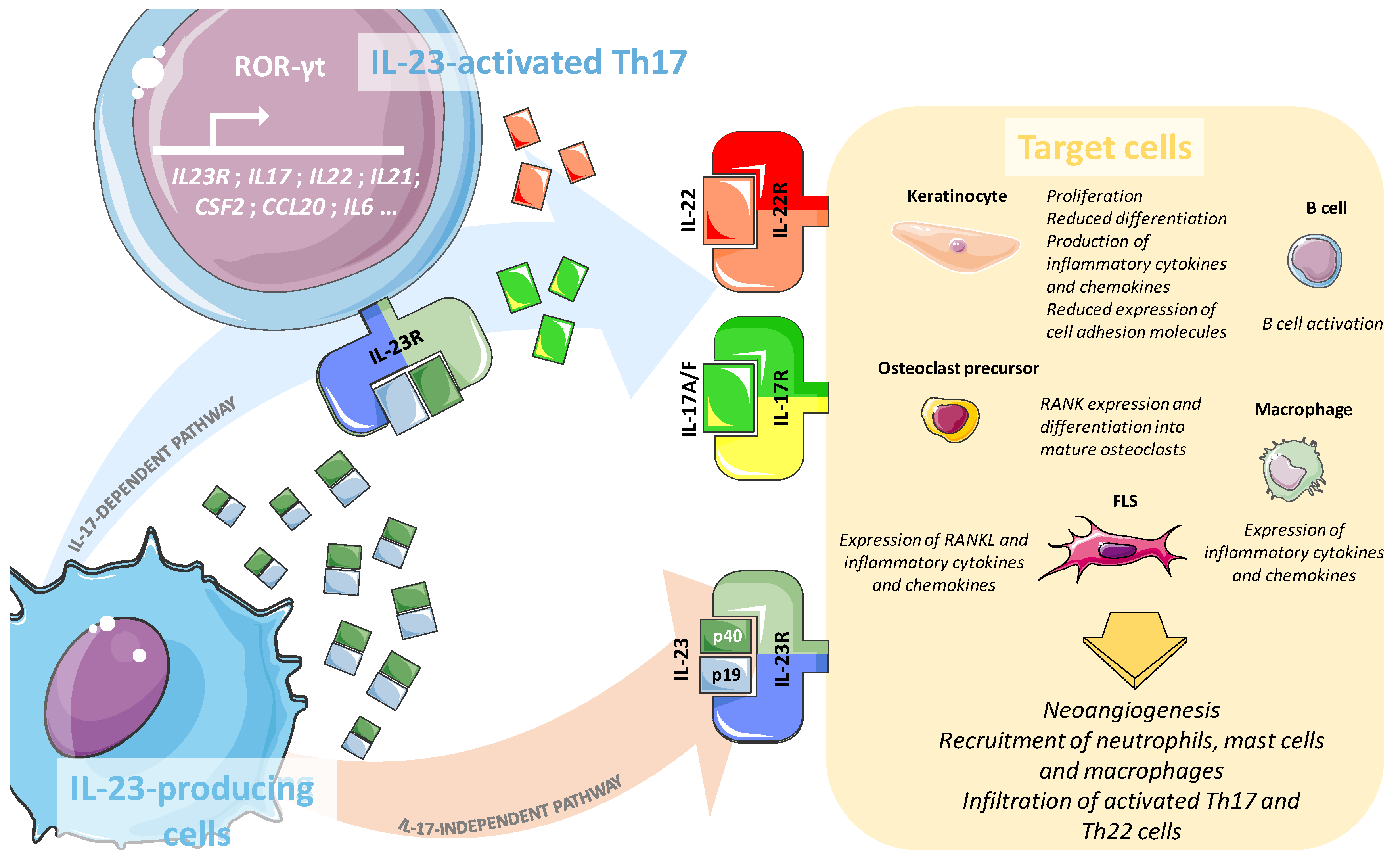
Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html

Inhibition Of Interleukin 12 And Or Interleukin 23 For The Treatment Of Psoriasis What Is The Evidence For An Effect On Malignancy Ergen 18 Experimental Dermatology Wiley Online Library
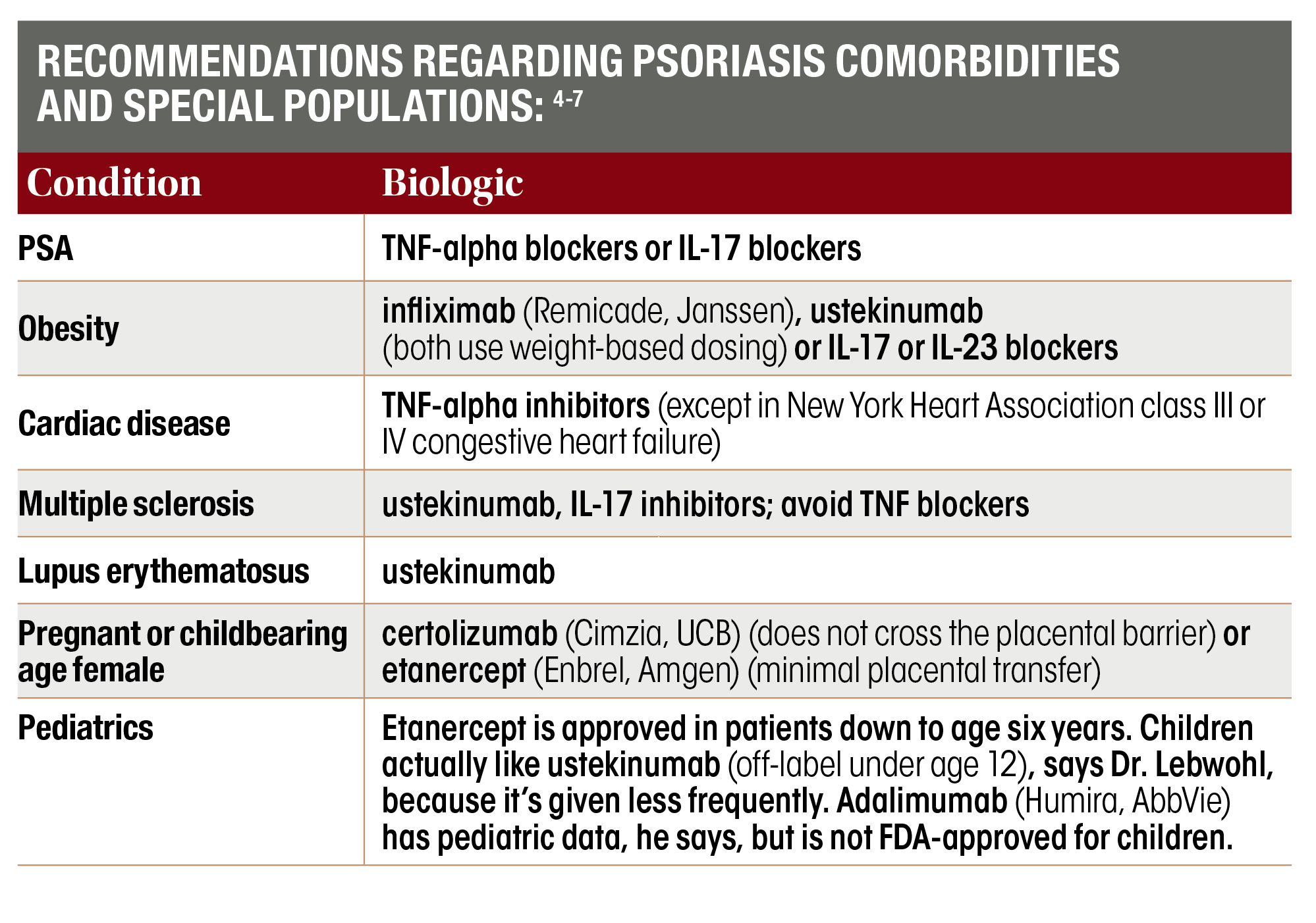
Considering Comorbidities In Psoriasis Treatment Dermatology Times And Multimedia Medical Llc
Ard Bmj Com Content Annrheumdis 78 8 1015 Full Pdf

Stat3 And Nf Kb Signal Pathway Is Required For Il 23 Mediated Il 17 Production In Spontaneous Arthritis Animal Model Il 1 Receptor Antagonist Deficient Mice The Journal Of Immunology

Full Text Spotlight On Risankizumab And Its Potential In The Treatment Of Plaque Ptt

Il 23 And Il 27 Levels In Serum Are Associated With The Process And The Recovery Of Guillain Barre Syndrome Scientific Reports

Tildrakizumab For The Treatment Of Psoriasis Immunotherapy

Interleukin 23 In Psoriasis Integrating New Therapies In The Current Treatment Landscape European Medical Journal

Il 23 Inhibitors For Psoriasis Therapeutic Cheat Sheet Next Steps In Dermatology

Guselkumab Versus Secukinumab For The Treatment Of Moderate To Severe Psoriasis Eclipse Results From A Phase 3 Randomised Controlled Trial The Lancet

Pdf Short Term Efficacy And Safety Of Il 17 Il 12 23 And Il 23 Inhibitors Brodalumab Secukinumab Ixekizumab Ustekinumab Guselkumab Tildrakizumab And Risankizumab For The Treatment Of Moderate To Severe Plaque Psoriasis A Systematic Review And

Psoriasis Pathogenesis And The Development Of Novel Targeted Immune Therapies Journal Of Allergy And Clinical Immunology

Interleukin 23 And Interleukin 17 Importance In Pathogenesis And Therapy Of Psoriasis

Therapeutic Applications Strategies And Molecules Targeting The Il 17 Th17 Pathway Musculoskeletal Key

A Receptor For The Heterodimeric Cytokine Il 23 Is Composed Of Il 12rb1 And A Novel Cytokine Receptor Subunit Il 23r The Journal Of Immunology
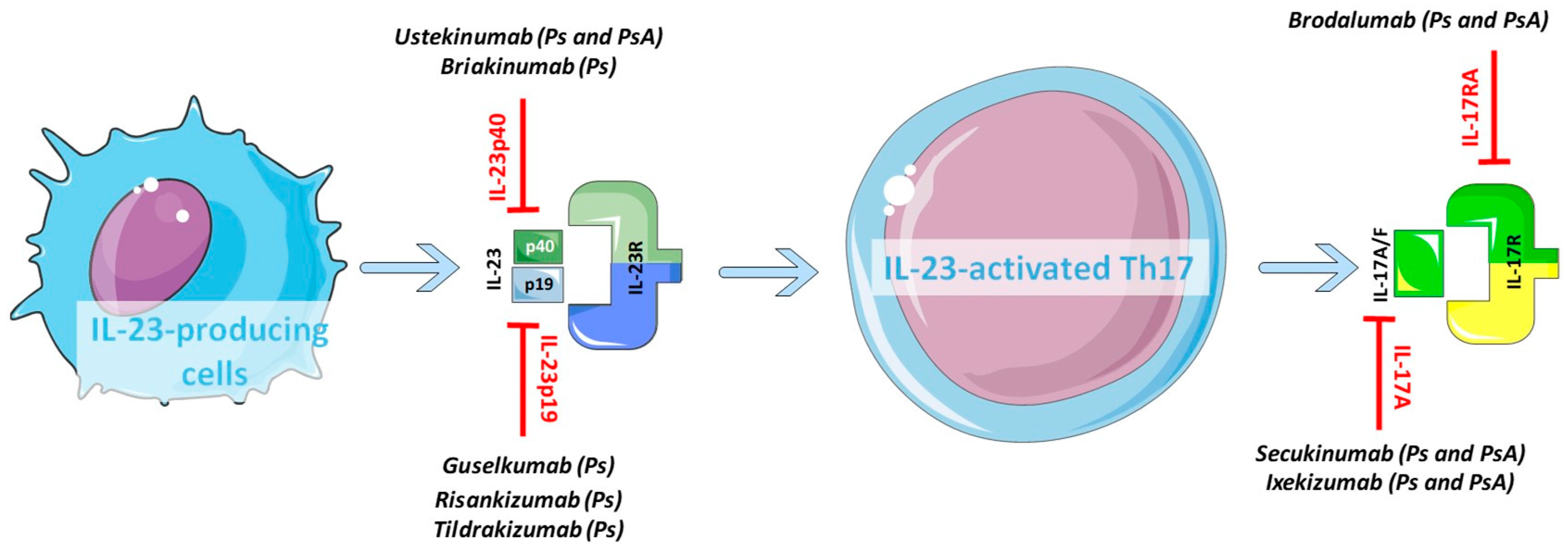
Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html
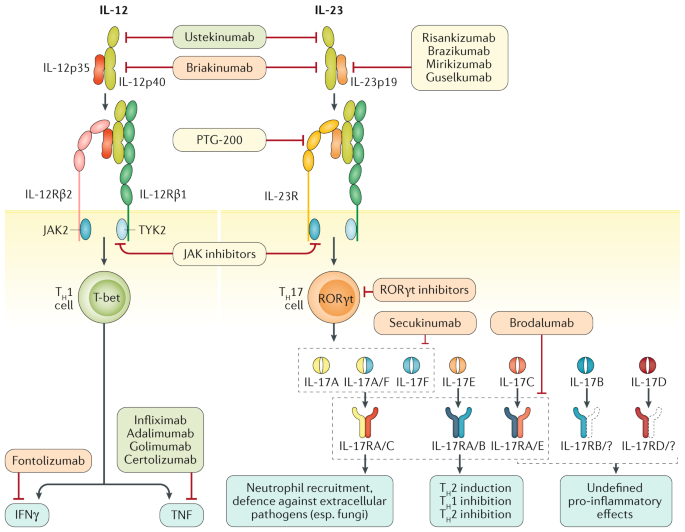
Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology

The Il 23 Th17 Axis In The Immunopathogenesis Of Psoriasis Sciencedirect

Il 23 Inhibitors For Psoriasis Therapeutic Cheat Sheet Next Steps In Dermatology
1
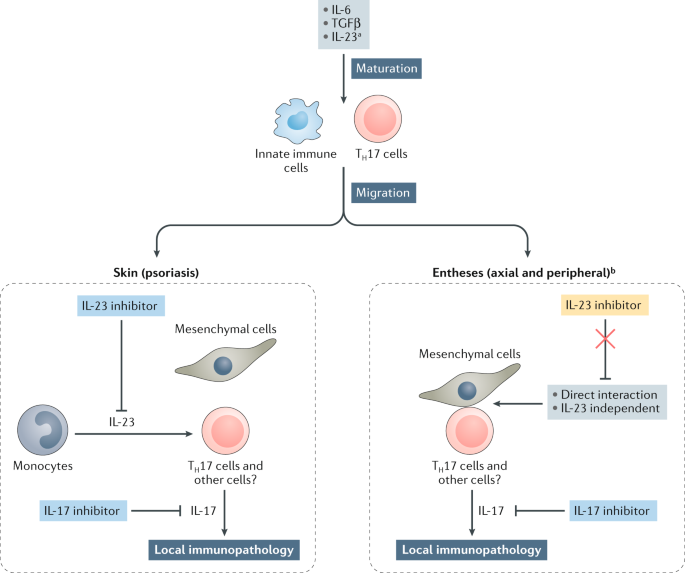
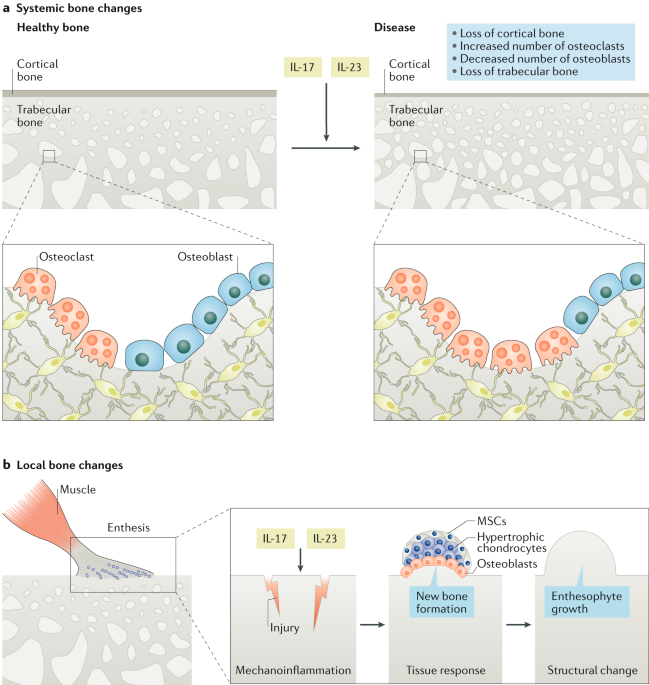
The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology

Il 23 Inhibition In Psoriasis Changing The Present Shaping The Future European Medical Journal
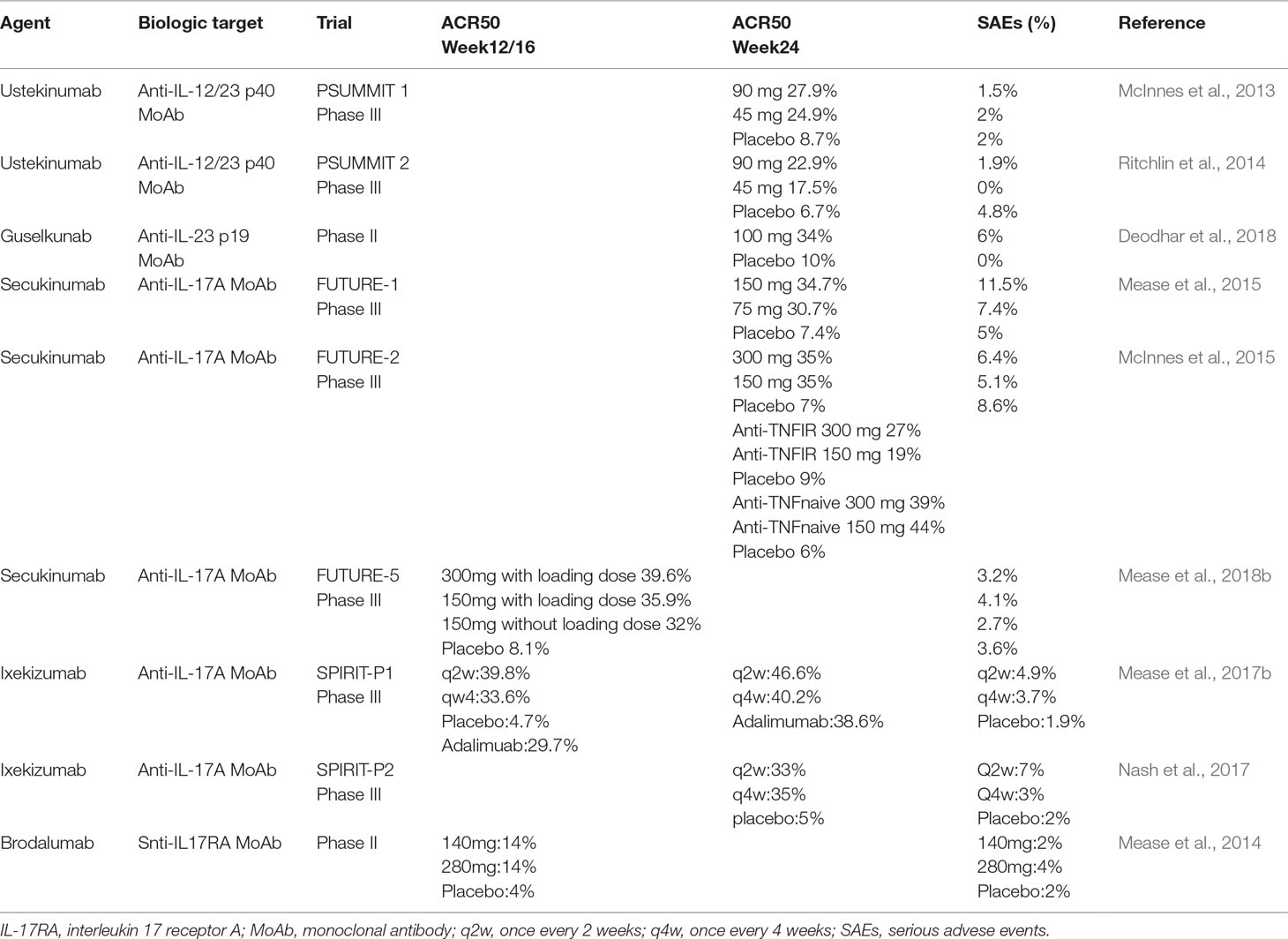
Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology
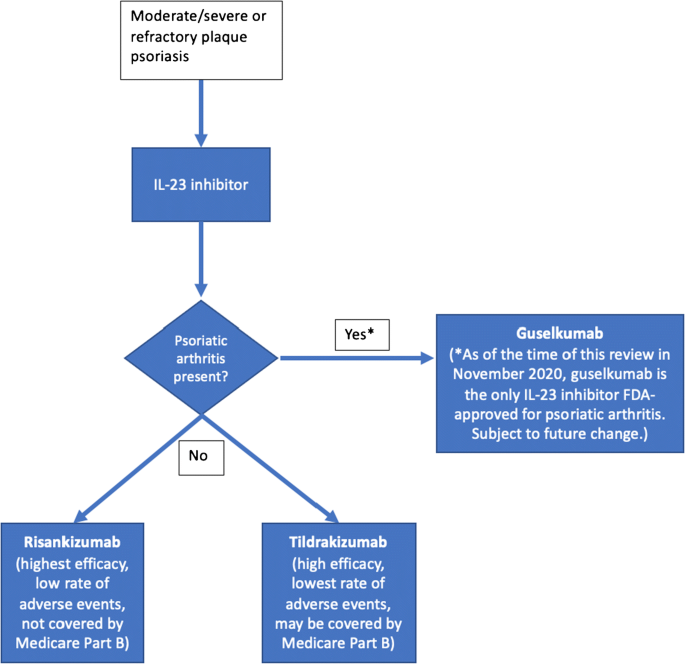
Advancements In Biologic Therapy For Psoriasis The Il 23 Inhibitors Springerlink
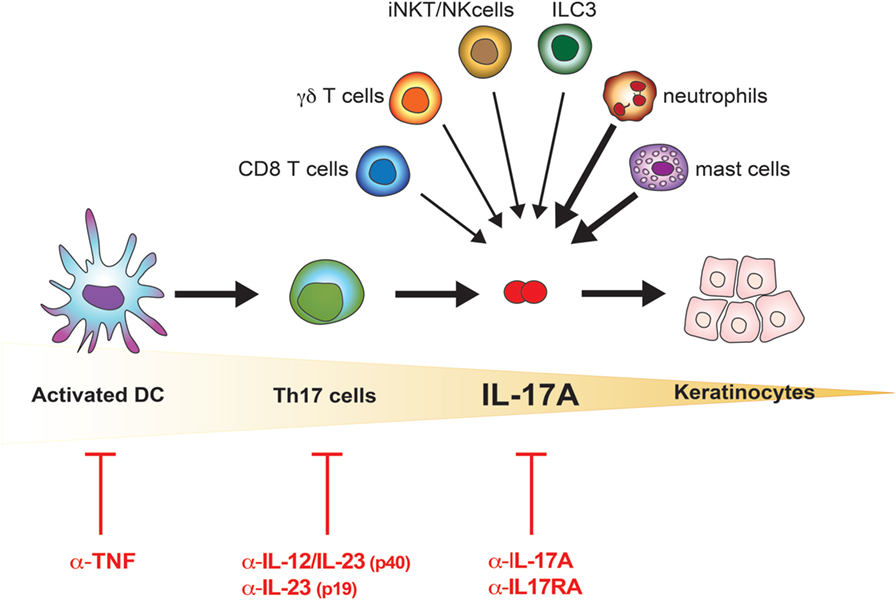
Frontiers The Il 17 Family Of Cytokines In Psoriasis Il 17a And Beyond Immunology
Q Tbn And9gcr3mhz6mcjy6jek4cw2jcumiwk Oyqmbe3r74bs4ois7cvvf9 O Usqp Cau

Biologic Therapies For Psoriasis Plastic Surgery Key

Shifting The Focus The Primary Role Of Il 23 In Psoriasis And Other Inflammatory Disorders Gooderham 18 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library

Figure 1 From Selective Interleukin 23 P19 Inhibition Another Game Changer In Psoriasis Focus On Risankizumab Semantic Scholar

Il 23 Inhibition In Psoriasis A Novel Approach To Convenient Consistent Clearance European Medical Journal
Www Tandfonline Com Doi Pdf 10 1080 x 17
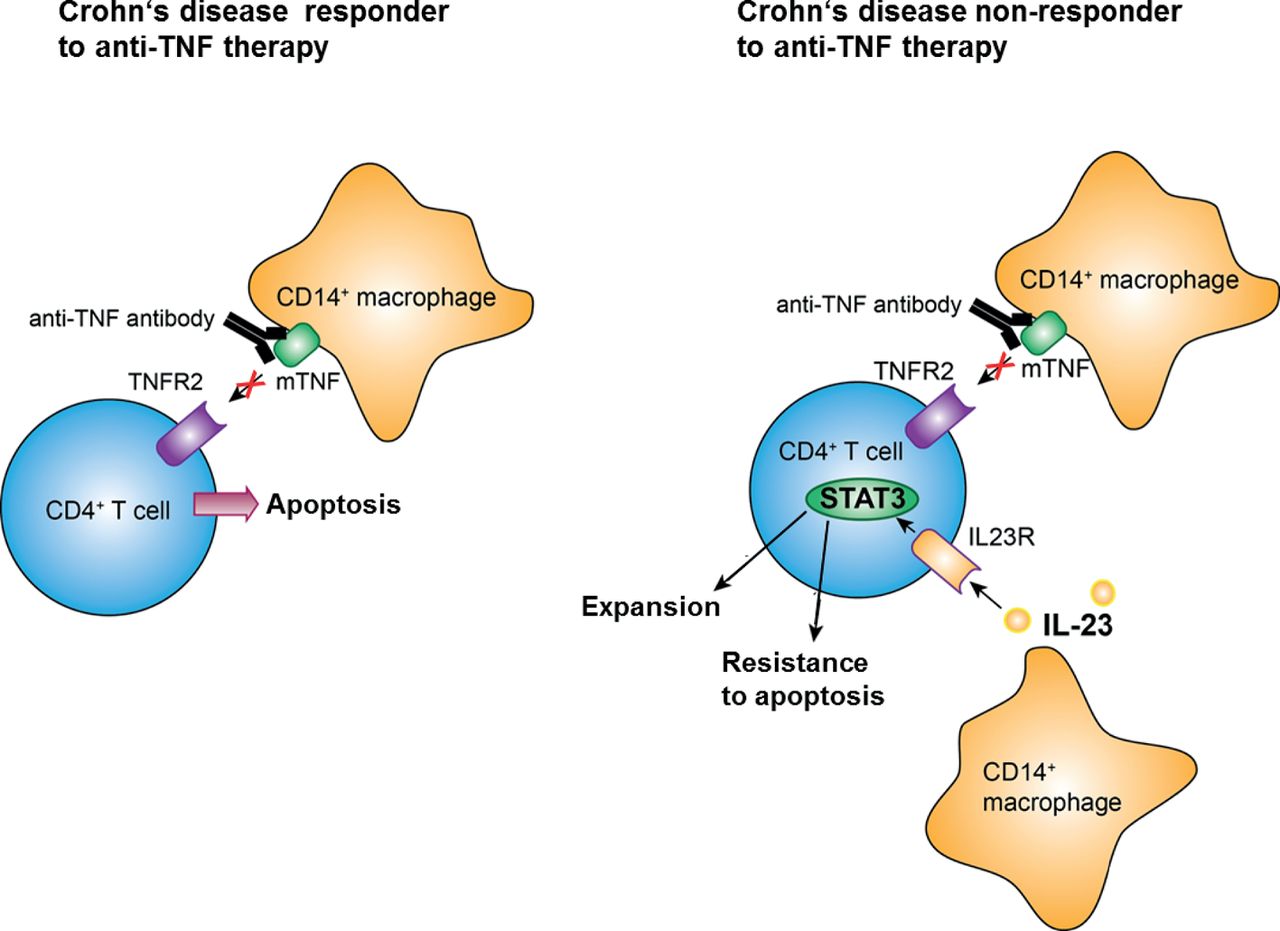
Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut

Interleukin 23 And Interleukin 17 Importance In Pathogenesis And Therapy Of Psoriasis

A Review Article On Brodalumab In The Treatment Of Moderate To Severe Plaque Psoriasis Immunotherapy

Skyrizi Risankizumab Rzaa Dosing Schedule

Irak1 4 Inhibitor Rigel Pharmaceuticals
Www Jaad Org Article S0190 9622 X Pdf

The Interleukin Il 23 Th17 Axis In The Immunopathogenesis Of Psoriasis Download Scientific Diagram
Novartis Gcs Web Com Static Files Ad03a2bf 041a 4190 A592 C91f185fb645
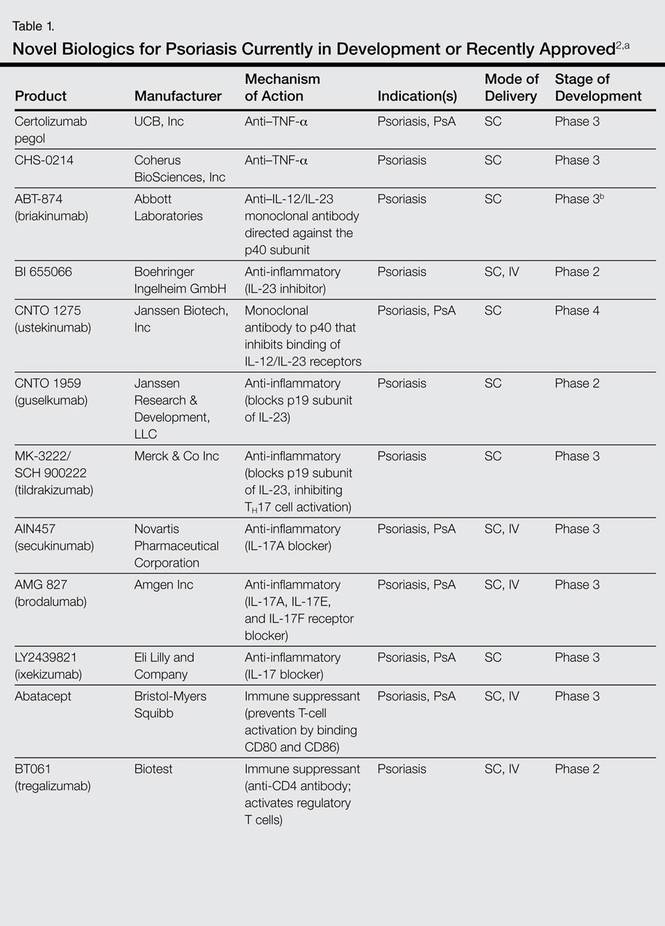
Novel Psoriasis Therapies And Patient Outcomes Part 2 Biologic Treatments Mdedge Dermatology

Interleukin 23 Inhibition As A Strategy To Treat Immune Mediated Inflammatory Diseases European Medical Journal

Positioning Anti Il 12 And Anti Il 23 Inhibitors Youtube

Inside The New Psoriasis Guidelines Part I The Dermatologist

Tofacitinib Attenuates Pathologic Immune Pathways In Patients With Psoriasis A Randomized Phase 2 Study Journal Of Allergy And Clinical Immunology
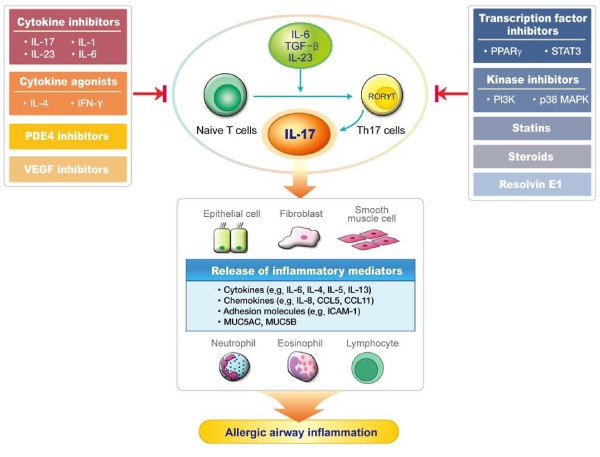
Interleukin 17 Regulation An Attractive Therapeutic Approach For Asthma Respiratory Research Full Text

Monoclonal Antibodies Inhibiting Il 12 23 And 17 For The Treatment Of Psoriasis Abstract Europe Pmc

New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram
Il 23 Signaling Regulation Of Pro Inflammatory T Cell Migration Uncovered By Phosphoproteomics

Biologic Therapy For Psoriasis Still Searching For The Best Target
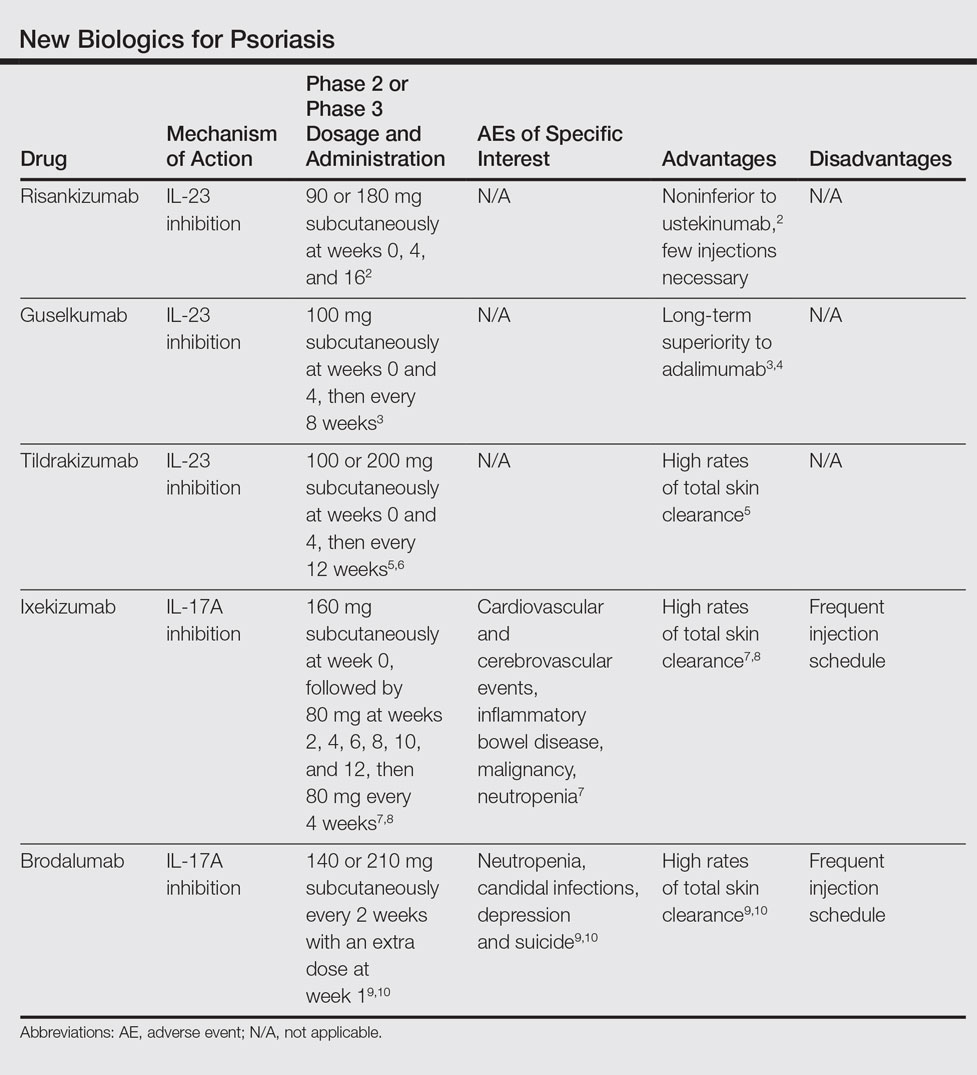
New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology

Interleukin 17 A Potential Therapeutic Target In Covid 19 Journal Of Infection
Risk For Development Of Inflammatory Bowel Disease Under Inhibition Of Interleukin 17 A Systematic Review And Meta Analysis

Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology

Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor



